The Food and Drug Administration (FDA) recently announced that two heart devices, the HeartMate II and HeartMate 3 Left Ventricular Assist System (LVAS), have been connected to hundreds of injuries and at least 14 deaths.
As a result of long-term buildup, these devices have been given the agency’s most serious recall classification. Safety advocates and medical experts are concerned about how approved medical device faults are managed and reported in light of the recall.
This continuous issue has resulted in 28 occurrences and 22 injuries. There have been no deaths reported. Medtronic had to recall 87,709 devices due to a similar issue in August 2022.
About the Abbott devices
Thoratec Corp., a division of Abbott Laboratories, manufactures the HeartMate II and HeartMate 3. Approximately 14,000 gadgets are thought to be under recall, however the two devices are not currently being pulled off the market.
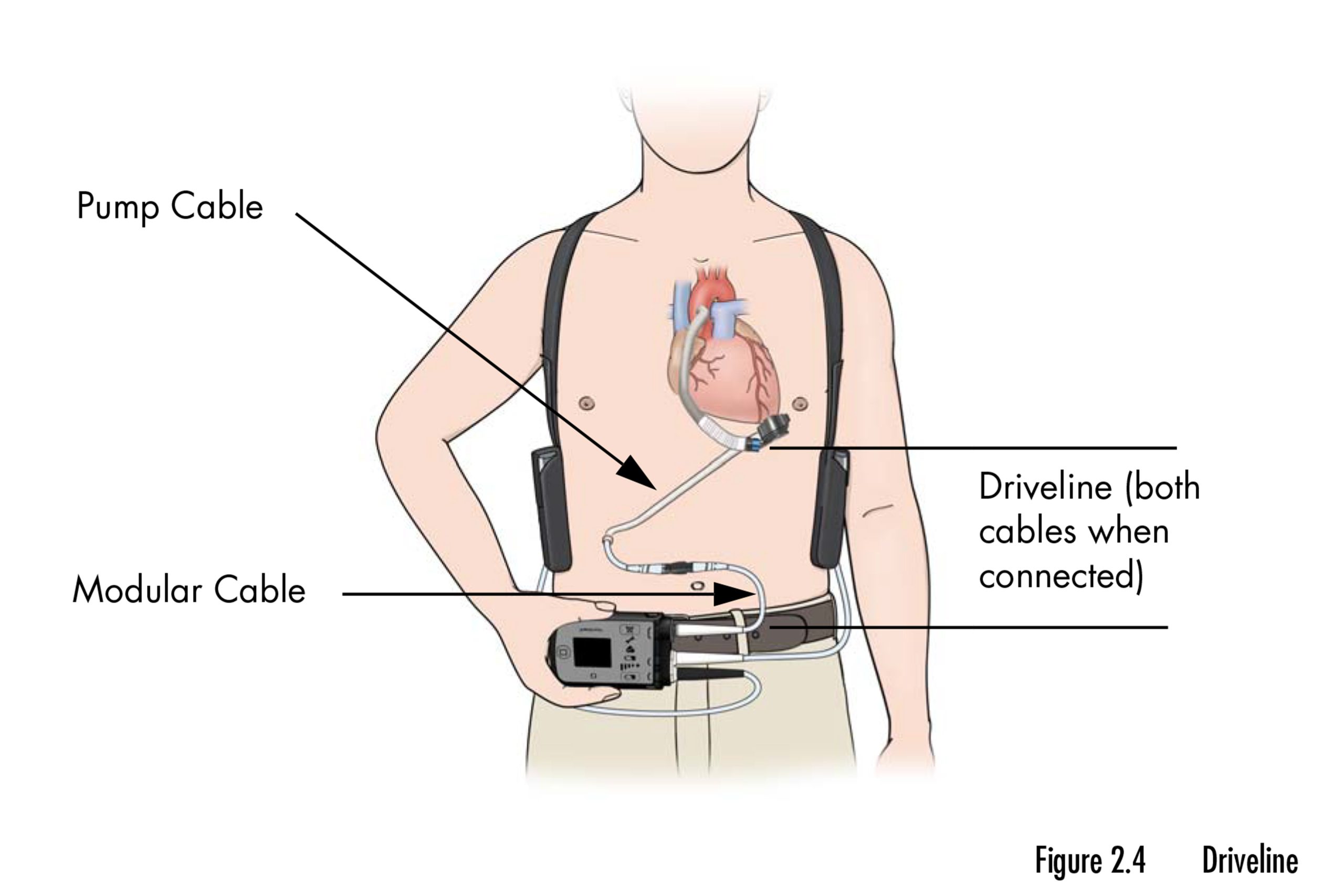
In a statement, the FDA stated, “The HeartMate II and 3 are used for both short- and long-term support in adult patients with severe left ventricular heart failure.” “It can be used while waiting for a heart transplant, to help the heart recover, or as a permanent solution when a transplant isn’t an option.”
The devices take the position of the left ventricle, the heart’s primary pumping chamber, in pumping blood. Blood flow from that compromised chamber is redirected and propelled into the aorta, where it is distributed throughout the body.
Amanda Hils, an FDA press officer, stated that the agency is working with Abbott to investigate the reported injuries and deaths and determine whether any action is required.

